Decoding Sunscreen Efficacy Testing
Recently, there is confusion in online social media regarding effectiveness and reliability of sunscreen products. The values stated on the labels and those measured by consumer protection agencies or influencers have shown varying results—some discrepancies are minor, while others are shockingly large. This has led to concerns and questions from consumers in several countries, including Thailand, about how well the products they choose can protect their skin. Can these products provide the level of protection indicated on the labels?
The doubts among consumers have resulted in content that uses various testing methods for marketing purposes, either to boast confidence in their own products or to discredit or attack competitors. Some lack comprehensive perspective, causing confusion among consumers and businesses. This article aims to clarify several points, including current testing standards and why the products may not provide the skin protection indicated on the labels, as well as ways to prevent these issues.
- How are the protection values on sunscreen labels determined?
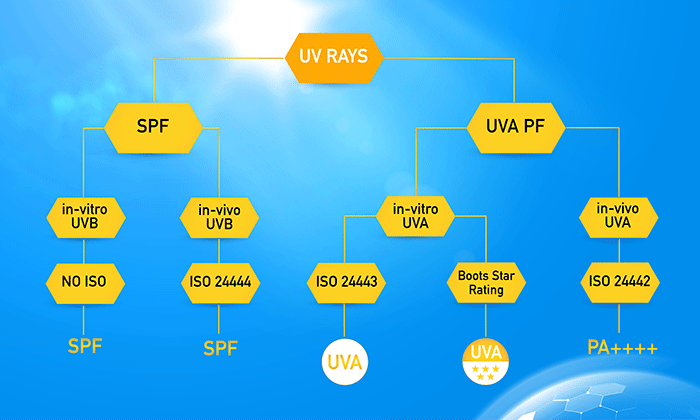
The labels of sunscreen products generally have SPF values indicating the level of protection against UVB ray, which is universally recognized. However, the indicators for UVA protection vary by region. In Asia, the PA (+) rating is used based on JCIA: 2012. In the EU, the UVA Seal/UVA mark is employed. In Australia/New Zealand and the US, broad-spectrum value is employed, while in the UK, the Boots Star® Rating is applied.
There are two evaluation methods of sunscreen product efficacy for registration and labeling purposes: In vivo (testing on human skin), and in vitro (testing on human skin substitutes and evaluated with specific instruments).
In vivo testing has been a long-standing method, but it has significant limitations as it is an invasive test. It raises ethical issues regarding humans exposed to radiation posing a risk of skin cancer. It is also more expensive and time-consuming. In contrast, in vitro testing has no ethical limitations, is less expensive, and can yield results in a shorter time.
Even though it seems that in vitro testing might be a better option in every aspect, in reality, in vitro testing still has issues with the materials used to replace human skin. Even the widely accepted and commonly used PMMA plates have concerns regarding the difficulty of spreading certain product types. It may not mimic the application on human skin. This affects the different formation of sunscreen product film layers and impacts the measured values. Therefore, both testing methods have their advantages and disadvantages.
In the past, sunscreen testing in each country based on vastly different standards, which posed obstacles to formulation development and made testing for registration cumbersome. However, in the present (2024), the latest revised ISO sunscreen testing standard has led major global markets such as Europe, Japan, Korea, Australia, and New Zealand to start implementing the same standards. Additionally, ISO 16217:2020 and ISO 18861:2020, the standard tests for measuring water resistance performance, also emerged.
Currently, the testing of UVB protection to determine SPF (Sun Protection Factor) is generally based on ISO 24444:2019 (in vivo).
The testing of UVA protection factor (UVA-PF) is based on ISO 24442:2022 (in vivo). It is currently accepted in Japan and South Korea. ISO 24443:2021 (in vitro) is widely accepted in Europe, Australia, New Zealand, and Asia, covering more than 60 countries. Many countries can use both standard, but some countries, like the UK, still use their own Boots Star® Rating standard. The US also employs their own standard with the US FDA regulations of 2021. Overall, ISO testing standards are slightly stricter than those of the US.
- How are the protection values on sunscreen labels determined?
As aforementioned above, currently, we do not have a standardized test for determining SPF using in vitro methods. It remains a significant challenge in the global cosmetics industry. This is also one of the reasons leading to the confusion among consumers and the Thai cosmetics industry.
In fact, SPF testing has always faced several issues, even with in vivo tests on human skin have been conducted for decades. Apart from the high costs and ethical concerns, there is also the issue of varying results from different testing centers. The same sunscreen formula tested by different laboratories for testing may yield different results. This can be attributed to several factors, such as the applying method, ethnicity, and skin redness factors. Additionally, measurement of minimal erythemal dose (MED) may vary based on human observation, leading to discrepancies among individuals.
According to issues, testing with ISO 24444:2019 has undergone several adjustments, such as clearer requirements for the distribution and application of the product, changing the standard for selecting test subjects from Fitzpatrick skin type to individual typology angle (ITA), adding reference sunscreen formulations for testing, and other adjustments. All to achieve more consistent test results.
As for in vitro SPF testing, we do not have an ISO standard method. The in vitro SPF tests often rely on methods that measure UVA protection in vitro. It cannot yet be perfectly correlated with in vivo SPF values. The results from each type of test can vary significantly; for example, an in vivo test might yield an SPF of 50, while the in vitro result might only result SPF of 15, for example.
The significant differences may stem from various factors and pose a challenge in finding a standard. I note that the current in vitro SPF tests are based on measuring the wavelengths that sunscreen agents can filter or block. It primarily focuses on the absorption of UV rays by sunscreen ingredients. The in vivo SPF test looks at occurring skin redness (minimal erythemal dose - MED). Current sunscreen formulations do not contain only sunscreen agents but may also include antioxidants or anti-inflammatory and anti-irritation ingredients to reduce or suppress skin redness. This could potentially lead to a higher SPF value, which might be one of the reasons for the discrepancy.
- The allowance for using in vitro SPF test in Thailand
While there is still no global standard for in vitro SPF testing and the ISO 24444:2019 must be used as the primary submission requirement. In Thailand, the guidelines for cosmetic registration allow submission of test results from manufacturer's laboratory, government agency, university, or testing unit using internationally accepted testing methods. In practice, Thailand has allowed submission of in vitro SPF test results for cosmetic registration. It may help reduce operational costs for businesses, facilitating the growth of the domestic cosmetics industry.
On the other hand, if clearer domestic standards for in vitro SPF testing are not established, we will have no reference for universal standard for evaluation procedures, processes, and equipment. This ambiguity can lead to the use of test results, which currently lack a unified standard, as content for marketing or to attack competitors. This may cause conflicts and confusion, negatively affecting consumer confidence in domestic brands, which is not beneficial for the Thai cosmetics business.
- Is in vivo testing more accurate and reliable than in vitro testing?
For testing UVA protection values, we currently have the ISO 24442:2022 (in vivo) and ISO 24443:2021 (in vitro) standards. It is accepted that the results of both testing methods are interrelated, both trustworthy and reliable when employed by a certified testing laboratory.
But for UVB protection testing, there is currently no ISO standard for in vitro SPF testing. We can employ in vitro tests to develop and create the most promising formulations before sending to in vivo testing according to ISO 24444:2019. This will provide more reliable values for registration and labeling. Manufacturers or business owners, who are serious about the credibility of their brands and products might consider the study, are suggested using the average obtained from 3-4 test centers to achieve more reliable SPF values of the labels.
- The new standard for sunscreen testing in the near future
From the collaboration of various parties, ISO is currently in the approval process of two additional sunscreen tests.
1. ISO 23675: in vitro SPF testing using UVR transmittance spectroscopy principles to test sunscreen products in emulsion and hydroalcoholic one-phase formulations.
2. ISO 23698: it is based on hybrid diffuse reflectance spectroscopy (HDRS) principle. The test combines both In vitro and noninvasive in vivo techniques, providing similar test results to human skin tests without any harm. This method can test either emulsion-based or single-phase alcohol-based sunscreen products, replacing ISO 24444:2019 (SPF in vivo) and ISO 24443:2021 (UVA-PF in vitro).
This new standard will benefit the development of effective sunscreen products, reducing the time and cost of development and testing while increasing accuracy and consistency of results. It is expected that this new ISO standard will be approved in the last quarter of 2025.

- Other reasons might cause lower protection values than the values indicated on the labels of sunscreen products?
The information provided indicates that even if the sunscreen is tested according to the correct standards, tested values, by someone or some agencies taken products from the market may match or do not match the labels. However, this is understandable because of the limitations of sunscreen testing as mentioned above.
But there are many cases where test results are vastly lower than the label stated, intentionally or unintentionally, such as:
1. Counterfeit cosmetics, illegal cosmetics, using fake labels, altering labels, non-registration, or registration fraud - consumers need to consider reputable brands and manufacturers, and purchase from trusted sources or official stores.
2. Excessive substances added in testing samples, but actual products do not apply registered formula and specifications on labels. This is a fraud originating from manufacturers, brand owners, or both parties.
3. Instability of the product formula; even if the formula is fully packed with sunscreen agents, the protection values may decrease if there are stability issues. They may arise from various factors, including:
- Using sunscreen agents with low photostability, which will gradually lose effectiveness over their shelf life and due to the storage conditions. The product may initially yield high test results. However, over time, if samples are randomly tested, it will be found that the results decrease and cannot protect the skin as indicated on the label.
- Separation of emulsion, such as oil separation. It affects user's feel and confidence, formation of the sunscreen film may be altered, and SPF values are affected. However, at least, the sunscreen remains in a usable form.
- Recrystallization of sunscreen can be observed as small crystal particles in the products. It feels rough on the skin when applied. It is a very serious issue caused by certain types of UV-absorbing agents. These gradients are often in powder form and need to be dissolved in the formulation. Recrystallization in the formulation will render such sunscreens completely ineffective in absorbing UV rays.
As above mentioned, product stability is extremely important. However, this issue can be controlled by starting with the selection of photostable sunscreen agents. If the product formulation is adjusted, it must also be considered that adding sunscreen agents to the formula without adjusting the solvent and other factors can accelerate recrystallization and affect overall product instability. Therefore, it is advisable to consult with specialized companies, particularly major sunscreen agent distributors, and conduct stability tests, including photostability tests, to reduce risks that could lead to product recalls and damage the brand’s reputation.
In summary, modern sunscreen products are becoming increasingly effective and safer due to the continuous development of new sunscreen agents and ingredients that enhance their efficacy. As well as the advancement of testing methods for greater effectiveness and reliability.
Regulation providers and enforcement authorities should primarily rely on internationally recognized standards. If there is any allowance to manufacture, clear standards should be established to serve as a common benchmark.
Cosmetic manufacturers and business owners should not overlook product testing, not just efficacy but also stability, which is essential for consumer safety and brand sustainability.
